|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||
|
|
|
Results from Spring 2004 Developmental Biology Laboratory at Swarthmore College Invagination was abnormal in embryos that developed in LiCl solutions. Embryos in the 60 mM solution died before fixation. A small number of embryos at 30 mM and 15 mM were successfully fixed, examined, and photographed. Embryos in the 15 mM solution displayed some exogastrulation and proportionally large archenteron formation (Figure 1). Sea urchin embryos that developed in 30 mM solution displayed abnormal gastrulation. Exogastrulation and distorted, globular form were common in the embryos (Figure 2). Sea urchins allowed to develop normally in ASW developed normal archenterons (Figure 3). 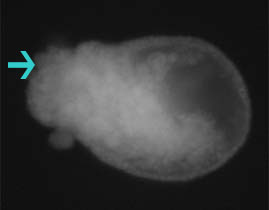
Figure 1. L. variegates embyro raised in 15 mM LiCl shows abnormal development. Arrow points to region of exogastrulation. Gut tissue is localized in the middle of the embryo.
Figure 2. Image of L. variegates embryo allowed to develop in 30 mM LiCl. Exogastrulation (arrow) and globular form are characteristics of abnormal archenteron development. Visualized vegetal and archenteron cells can be found on all parts of the embryo.
Figure 3. L. variegates embryo allowed to develop in ASW solution displayed normal archenteron development. Visualized vegetal cells have developed into the archenteron and the hindgut. The results of this experiment are in agreement with reported results. Abnormalities in archenteron development were observed in embryos that developed in LiCl solutions. The death of all embryos in 60 mM solution of LiCl was unexpected; it is speculated that LiCl effects were too strong for development for a significant length of time. Lihium chloride is a teratogen that has long been known to increase the proportion of sea urchin embryo cells that contribute to the archenteron (Horstadius, 1973). LiCl disrupts typical B-catenin expression (Cameron and Davidson, 1997). Increased nuclear B-catenin is acheived by LiCl treatment. Endo 16, a cell surface molecule involved in gastrulation, is recruited by cells treated with lithium chloride (Nocente-McGrath et al., 1989). The increased number of Endo 16 cells in the vegetal plate disrupts archenteron gastrulation. Gastrulation utilizes cellular movement and shape change. Cellular migration requires a dynamic adhesion system for specific changes in cellular shape and cell-cell contact while allowing the cellular sheet to remain intact during invagination. During gastrulation and convergent-extension B-catenin displays significant changes in localization in conjunction with morphogenic events. This suggests that B-catenin might be involved in adhesion signaling (Miller and McClay, 1997). Disruption in adhesion processes will disrupt cell flexibility and mobility. Extensive cellular movement and shpae change is responsible for the archenteron elongation (Ettensohn, 1985). Disrupted archenteron development during treatment with LiCL clearly link these factors.
References
Gilbert, S. F. Developmetnal Biology. Sinauer Associates, Inc. 1997. Gilbert, S. F. 2003. Developmental Biology, 7th ed. Sinauer Associates, Inc., Publishers, Sunderland Massachusetts, pp. 227-231. Hostadius, S. Experimental Embryology of Echinoderms. Clarendon, Oxford, UK. 1973. Klein, P.S., and Melton, D.A. 1996. A Molecular Mechanism for the Effect of Lithium on Development. Proc. Natl. Acad. Sci. USA 86, 3669-3673. Logan, C.Y., J.R. Miller, M.J. Ferkowicz, and D.R. McClay. 1999. Nuclear B-catenin is required to specify vegetal cell fates in the sea urchin embryo. Dev. 126: 345-357. Miller, J.R. and D.R. McClay. 1997. Changes in the Patterns of Adherens Junction-Associated B-catenin Accompany Morphogenesis in the Sea Urchin Embryo. Dev. Bio. 192: 310-322.
|
||||||||
Last Modified: 1 May 2004
[Lab Protocols | Students | Cebra-Thomas | Course | Links ]